Molecular assembly is the self-arrangement of molecules or molecular groups under a limited condition, which is an important area in supramolecular chemistry. By employing intermolecular interactions, molecular assembly method can be applied in various areas in matter science research, leading to a very active multidisciplinary area. The research that how simple molecules to form hierarchical ordered aggregation structures through non-covalent interactions can not only provide a new way to explore new assemblies, but also paving an efficient route to design new materials. We use molecules or molecular complex as the fundamental building blocks and our main research focus on exploring the interactions and synergistic effect of different molecular building blocks. Our final purpose is to construct novel assembling structures and develop supramolecular materials with potential applications, based on the rational combination of molecules and aiming at different scientific questions.

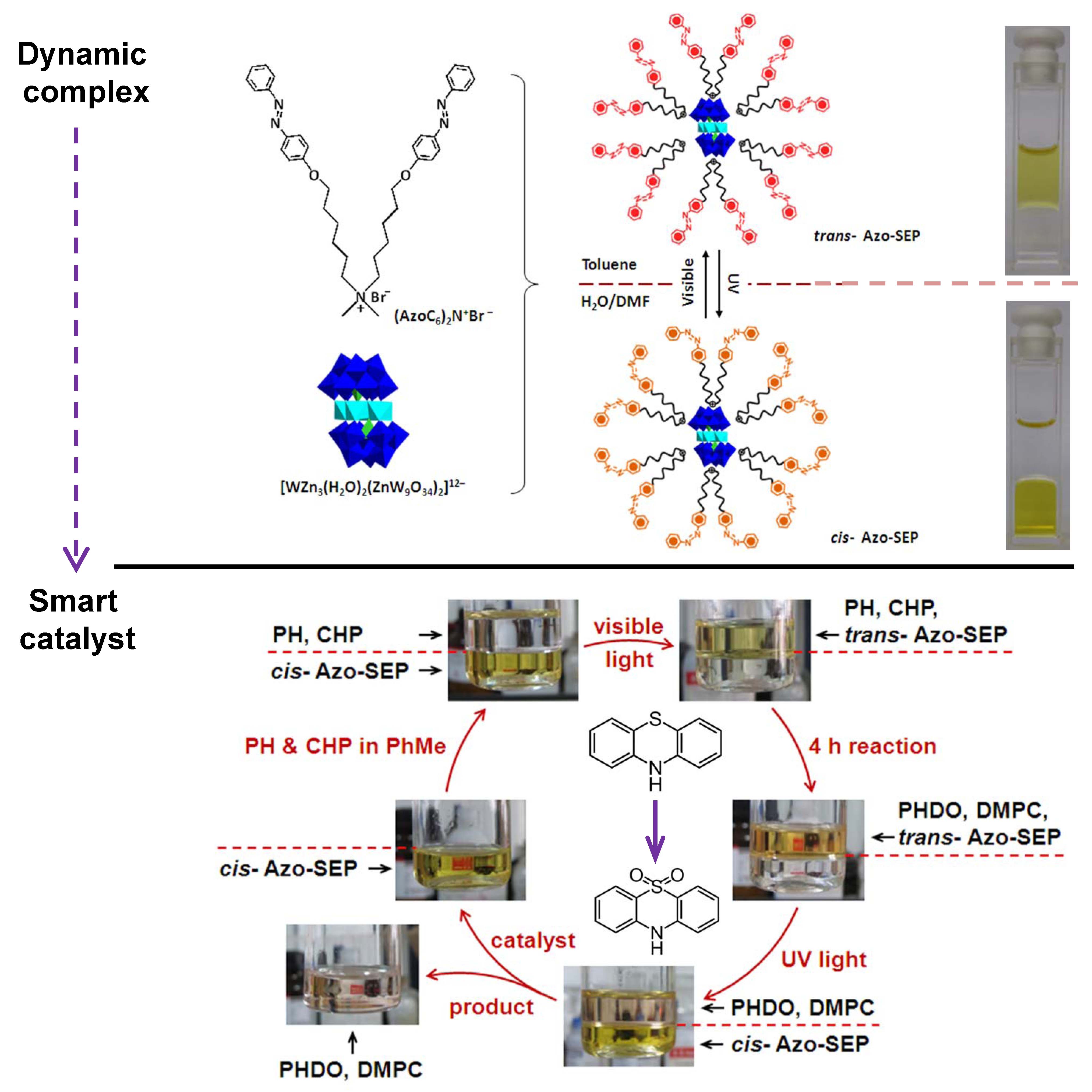
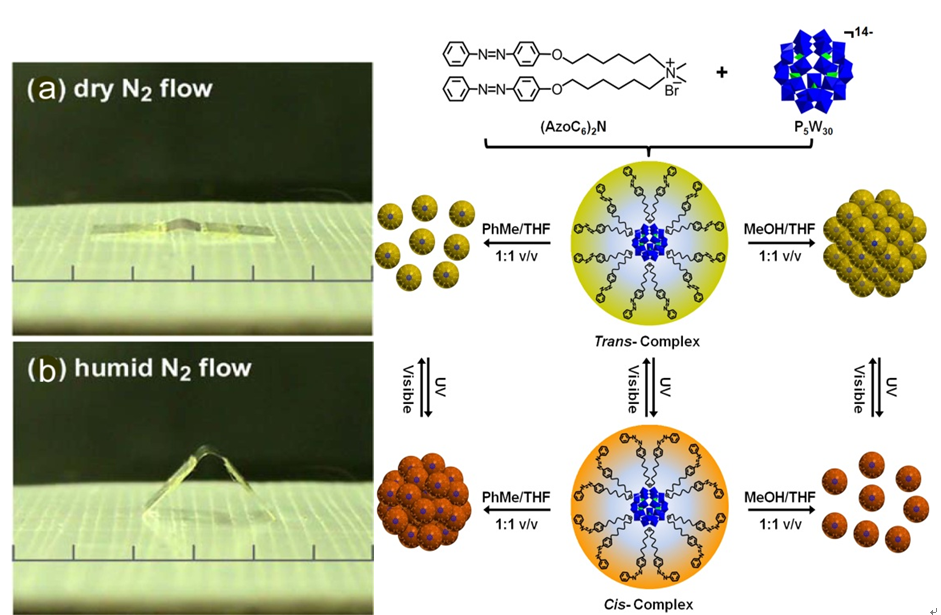
A new photosensitive complex has been fabricated by graftingazobenzene groups onto catalytic polyoxometalate cluster via ionic interactions. The complex can only dissolve in weak polar solvent because of the hydrophobic out-layer. However, it readily transfersintotraceDMF-containing aqueous solution under UV-irradiation, which opens up the significant potential in the development of smart catalyst witha homogeneous catalysis and heterogeneous separation.The catalytic oxidation of dye molecules in organic solvent and the subsequent separation of the catalytic complex can be realized with the help of UV-irradiation. The catalytic recycle can be performed several times. The outstanding advantage of this assembly is the value-addedcombination of photo-sensitiveligands and the catalytic cluster. (J. Am. Chem. Soc. 2013, 135, 14500-14503). It is envisioned that the dynamic feature of the complex may be further explored as multi-phase catalysis and multi-phase separation system.
Acridines as an important class of heteroaromatic compounds have attracted considerable interest because of their novel hetreocycle chemistry, broad range of properties, and industrial applications. Many efforts have been focused on the development of synthetic methods for the construction of a variety of acridine derivatives. Most of these known synthetic methods require either harsh reaction conditions, such as high temperature, strongly basic or acidic media, or difficultly obtained starting materials. Therefore, it is highly desirable to develop new synthetic methods for acridine derivatives under relatively mild conditions. We report an efficient method to synthesize acridine derivatives and related polycyclic aza-aromatic compounds, such as benzo[j][1,7]phenanthroline, dibenzo[b,j][4,7]phenanthroline, dibenzo[b,j][1,7]phenanthroline, and acridino[4,3-c]acridine, from a ZnCl2-promoted cyclization reaction of o-arylaminophenyl Schiff base compounds. (Org. Lett. 2014, 16, 18.)
By using the antireflective silicon nanocones arrays as SALDI substrate for small molecules analysis, we investigated the role of the absorbed laser energy and its distribution in the laser desorption/ionization process. The absorbed laser energy can be channeled completely into desorption/ionization of analytes by optimizing the surface features of antireflective structure. The optimized silicon nanocones array exhibits the excellent desorption/ionization performance for detecting peptide, amino acid, drug molecule, and carbohydrate with little or no interference in the low mass region. The detection limit of bradykinin is about 10 fmol with the signal to noise (S/N) ratio value of 11.39. The value of the linear regression R2 of the calibration curve for the glucose in human urine sample is 0.9992. Furthermore, the practical application of this method is demonstrated by the successful analysis of glucose in urine sample from a diabetic patient.
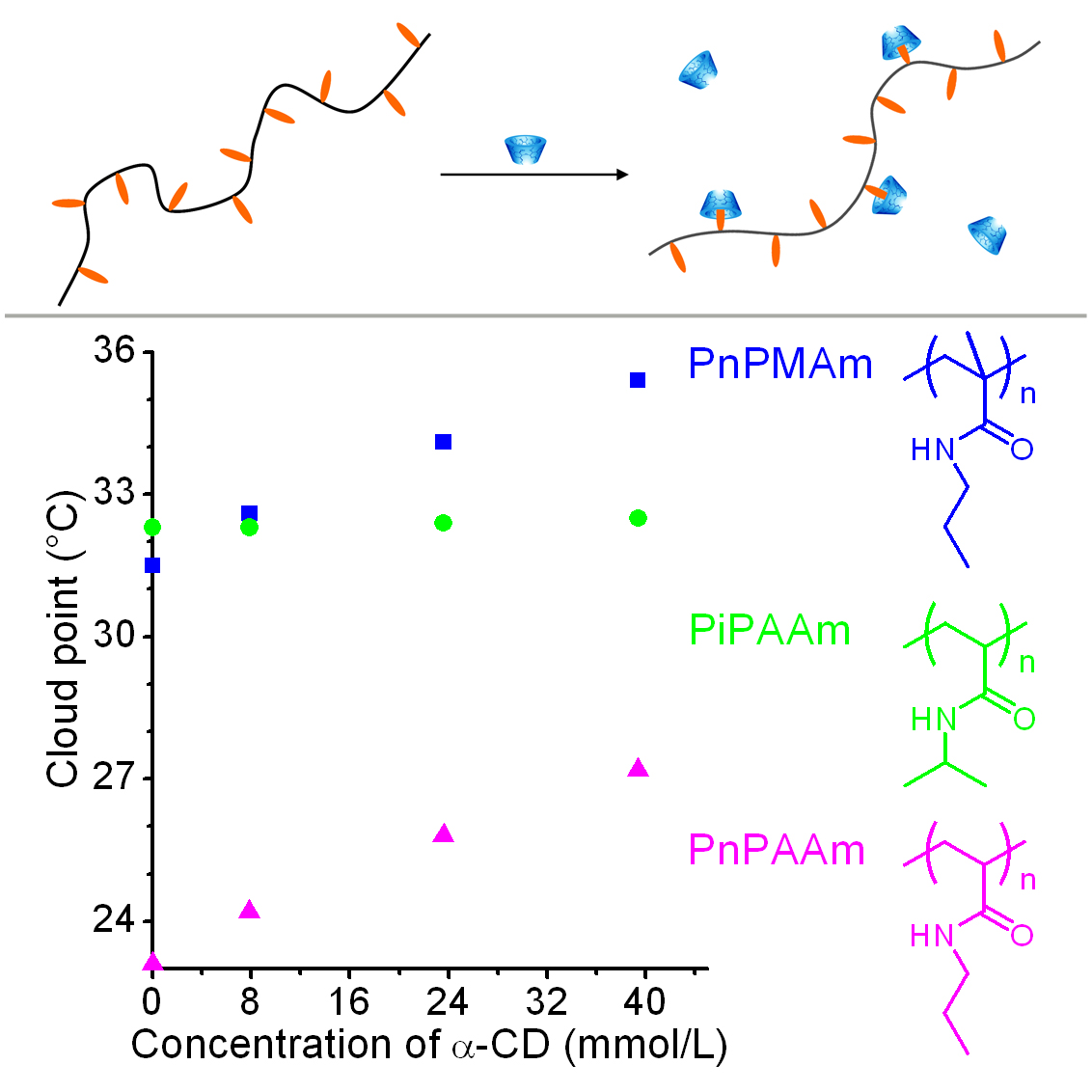
Stimuli-responsive polymers are polymers that have one or more properties that can be significantly changed in a controlled fashion by an external stimulus, such as temperature, humidity, pH, and light irradiation. Some thermosensitive homopolymers have hydrophobic side groups, egpoly(N-propylacrylamide) (PnPAAm). We found that the side groups can form host-guest complex with cyclodextrin, leading to an increase in cloud point of polymer solutions.Based oninclusion complexation equilibrium, we deduced an equation,which clearly described the relationship between cloud point andconcentration of cyclodextrin. Some thermosensitive homopolymers have polar side groups, such as poly(N-vinylcaprolactam) (PVCL) and poly(2-hydroxypropyl acrylate) (PHPA). Hydrogen-bonded layer-by-layer films of PVCL and PHPA were assembled at a constant temperature.When the film was immersed in purified water at a temperature lower than the assembly temperature, it can be dissolved.Such thin films with temperature-responsive solubility might be used as adissolvable film or a smart capsule.